Estradiol sulfamate (E2MATE; developmental code names J995, PGL-2, PGL-2001, ZK-190628, others), or estradiol-3-O-sulfamate, is a steroid sulfatase (STS) inhibitor which is under development for the treatment of endometriosis. It is the C3 sulfamate ester of estradiol, and was originally thought to be a prodrug of estradiol. The drug was first synthesized as an STS inhibitor along with its oxidized version estrone 3-O-sulfamate (EMATE) in the group of Professor Barry V L Potter at the University of Bath, UK, working together with Professor Michael J Reed at Imperial College, London and was found to be highly estrogenic in rodents. Such aryl sulfamate esters were shown to be "first-in-class" highly potent active site-directed irreversible STS inhibitors. Compounds of this class are thought to irreversibly modify the active site formylglycine residue of STS. The drug shows profoundly reduced susceptibility to first-pass metabolism relative to estradiol, and was believed to be the first "potent" estradiol prodrug to be discovered. It was clinically investigated for possible use as an estrogen for indications like hormonal contraception and menopausal hormone therapy. However, it showed no estrogenic effects in women. The potent non-estrogenic clinical STS inhibitor Irosustat (STX64/667-Coumate) was used to explore the possibility that STS might be responsible for the hydrolysis of estrogen sulphamates. Results demonstrated convincingly that STS is the enzyme responsible for the removal of the sulfamoyl group from estrogen sulfamates and has a crucial role in regulating the estrogenicity associated with this class of drug. Thus, STS inhibition blocks the conversion of E2MATE into estradiol and thereby abolishes its estrogenicity in humans. Irosustat has completed a number of clinical trials in oncology as an STS inhibitor currently up to Phase II.
 This DRUG has not been minted yet.
This DRUG has not been minted yet.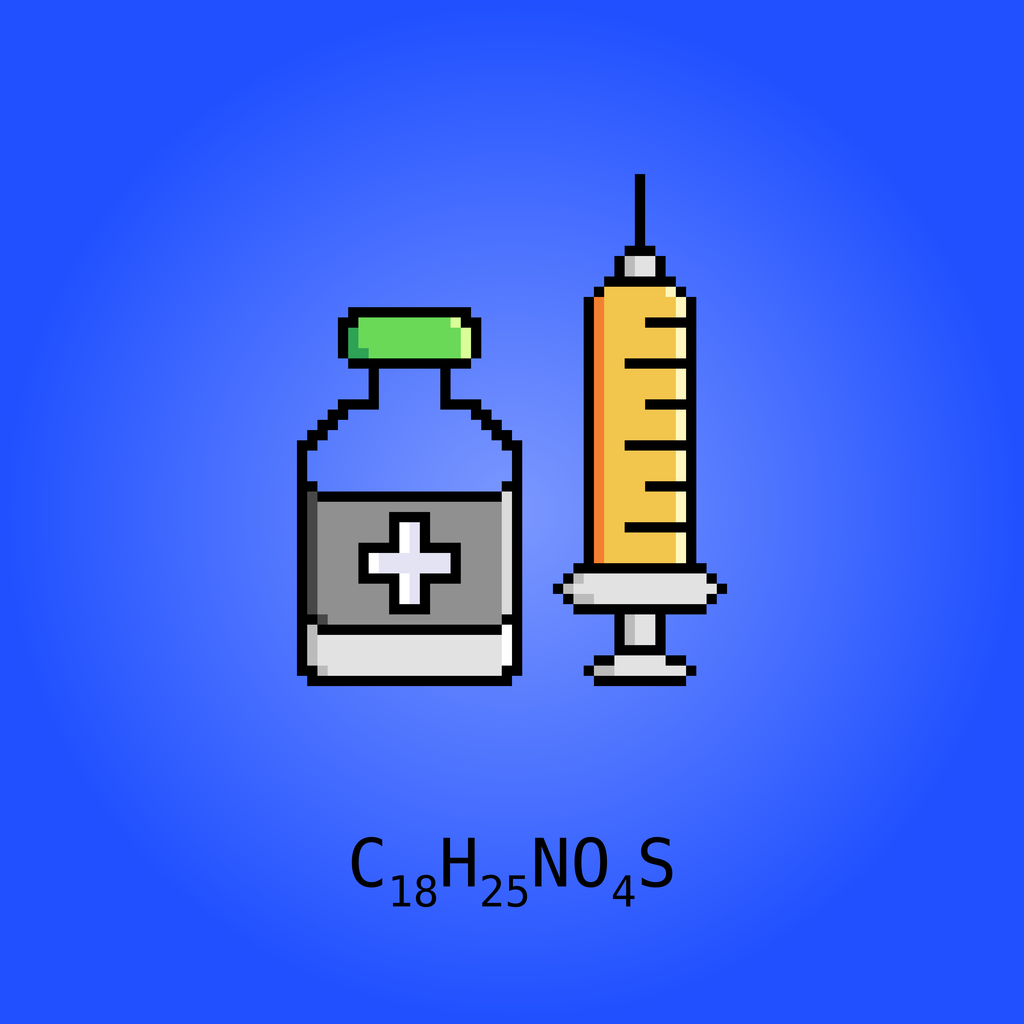
 on Earth by F. Boffa and T. Calemme
on Earth by F. Boffa and T. Calemme